The density of every band was estimated using the scanner GS 800 and evaluation system Quantity OneTM from BioRad Laboratories, Liquid chromatography electrospray tandem mass spectrometry and database examination For mass spectrometry analysis PC12 cell homogenates were separated by SDS Web page and digested in situ by trypsin as previously described, In particular, stick to ing SDS Page, each and every lane was lower in 2 mm bands and de stained in 0. 1% trifluoroacetic acid. acetonitrile one.1 prior to drying. Gel pieces had been rehydrated with trypsin remedy, and incubated overnight at 37 C. Peptides had been extracted from the gel using 0. 1% trifluoroacetic acid. acetonitrile one.one. The material was dried, resuspended in 10 uL 0. 3% v v formic acid and desalted working with Zip Tip C18 in advance of mass spectrometric examination. Samples were separated by liquid chromatography utilizing an Ultimate 3000 HPLC, Buffer A was 0.
1% v v formic acid, 2% acetonitrile, buffer B was 0. 1% formic acid in aceto nitrile. Chromatography selleck was performed utilizing a PepMap C18 column, The gradient was as follows. 5% buffer B, 5% 40% B, 40% 50% B 95%B at a movement rate of 1. two uL min. Mass spectrometry was performed using a LTQ Orbitrap Velos equipped having a nanospray source, Eluted pep tides were immediately electrosprayed more info here into the mass spec trometer by a typical non coated silica tip utilizing a spray voltage of 2. eight kV. The LTQ Orbitrap was operated in good mode in data dependent acquisition mode to immediately al ternate amongst a full scan within the Orbitrap and subsequent CID MS MS in the linear ion trap of the twenty most extreme peaks from total scan. Two replicate evaluation of every sample had been carried out. Data acquisition was managed by Xcalibur 2. 0 and Tune 2.
4 computer software, Looking for nitrated proteins towards the rat NCBInr database was carried out working with the Sequest internet search engine contained from the Prote ome Discoverer 1. 1 computer software, The next parameters have been made use of. 10 ppm for MS and 0. 5 Da for MS MS tolerance, carbamidomethylation of 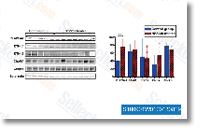 Cys as fixed modification, Met oxidation, Tyr nitra tion, Trp nitration and Ser Thr Tyr phosphorylation as variable modifications, trypsin as protease, False Discovery Charge for peptides 5%, nitrated peptides recognized amongst the Rank one peptides. Effects and discussion Substrate characterization Figure 1 report the AFM characterization of glass and flat TiO2 substrates. Poly L Lysine coated glass has a calculated rms roughness of 0. 271 0. 020 nm, whereas flat TiO2 films present a rms roughness of 0. 229 0. 004 nm. Figure one present SEM and AFM pictures of cluster assembled ns TiO2 movies with roughness of 20. 2 0. 5 nm and 29.
Cys as fixed modification, Met oxidation, Tyr nitra tion, Trp nitration and Ser Thr Tyr phosphorylation as variable modifications, trypsin as protease, False Discovery Charge for peptides 5%, nitrated peptides recognized amongst the Rank one peptides. Effects and discussion Substrate characterization Figure 1 report the AFM characterization of glass and flat TiO2 substrates. Poly L Lysine coated glass has a calculated rms roughness of 0. 271 0. 020 nm, whereas flat TiO2 films present a rms roughness of 0. 229 0. 004 nm. Figure one present SEM and AFM pictures of cluster assembled ns TiO2 movies with roughness of 20. 2 0. 5 nm and 29.
Igf Protein
Approximately 98% of IGF-1 is always bound to one of six binding proteins (IGF-BP)
